The Unique Triple-Action Formulation
revyve® Antimicrobial Wound Gel addresses the first 3 steps in the DIME wound healing framework

revyve® Antimicrobial Wound Gel is indicated for the use on:
- Partial and full thickness wounds
- Leg ulcers
- Large surface area wounds
- Pressure ulcers
- Surgical incisions
- 1st and 2nd degree burns
- Diabetic foot ulcers
”“I tried it on a nursing home patient with a stage 4 pressure ulcer with large exudate and pain. Amazing response with reduction of both exudate and pain after only a few dressing changes.”
Dr. Noland, Lakeview Family Health Team, Colborne, Ontario, Canada*
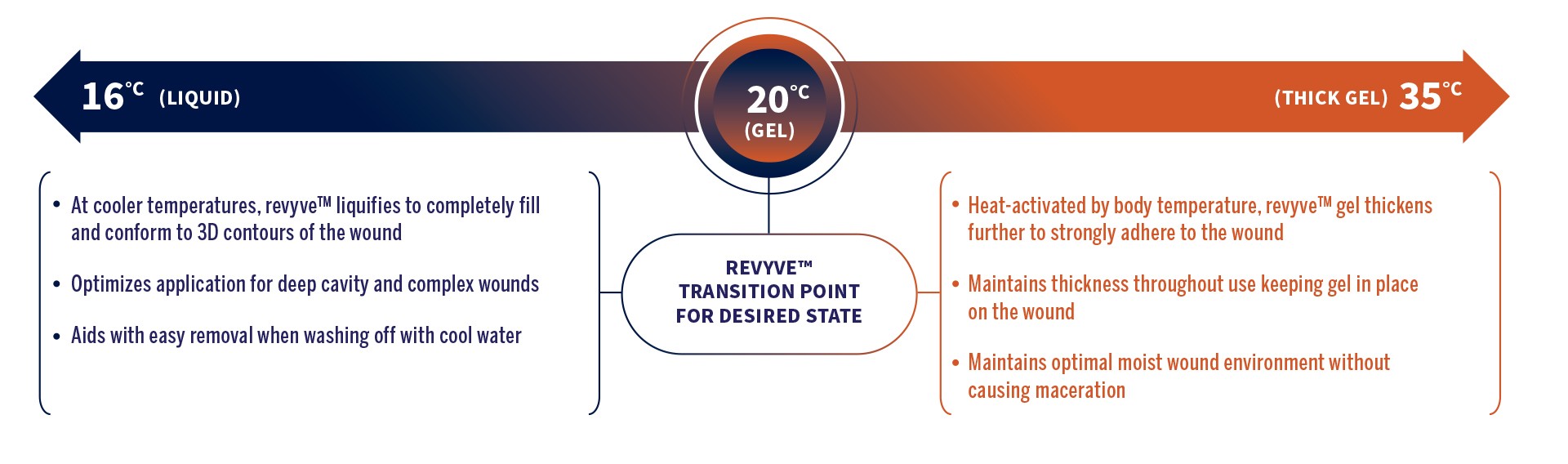
Novel Non-Aerosol Spray Application
- Zero-contact treatment that can be rapidly applied to traumatic, large surface and hard to reach wounds.
- Thick gel spray that strongly adheres to and stays in place on wounds.
- Designed for painless delivery and removal of gel
- Patent Pending
Spray gel currently under review with Health Canada
Biofilm Impaired Healing is the Largest Unresolved Problem in Wound Care
Over 70% of burns and 80% of chronic wounds have a biofilm layer. Recent data suggests that all chronic wounds have biofilm on at least part of the wound bed.
Biofilm formation can make bacteria up to 1,000 times more resistant to antibiotics, antimicrobial agents, disinfectants and the host immune system and are one of the main contributors to antibiotic resistance. The NIH estimates that 80% of all known human infections are associated with biofilms.
revyve® Antimicrobial Wound Gel is effective against:
GRAM-NEGATIVE BACTERIA
Escherichia coli
Acinetobacter baumannii
Klebsiella pneumoniae
Pseudomonas aeruginosa
GRAM-POSITIVE BACTERIA
Staphylococcus aureus
Methicillin Resistant Staphylococcus aureus
Enterococcus faecalis
Streptococcus pyogenes
Cutibacterium acnes
FUNGI
Candida albicans
About Kane Biotech
Kane Biotech is a biotechnology company engaged in the research, development and commercialization of technologies and products that prevent and remove microbial biofilms.
(1). Asati S, Chaudhary U. Prevalence of biofilm producing aerobic bacterial isolates in burn wound infections at a tertiary care hospital in northern India. Ann Burns Fire Disasters. 2017 Mar 31;30(1):39–42. (2). Malone, M., Bjarnsholt, T., McBain, A.J, James GA, Stoodley P, Leaper D, Tachi M, Schultz G, Swanson T, Wolcott RD. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. Journal of Wound Care. 2017 Jan 2;26(1):20–5. (3). Goswami, A.G., Basu, S., Banerjee, T. et al. Biofilm and wound healing: from bench to bedside. European Journal of Medical Research 2023 28, 157. (4). McCarty SM, Percival SL. Proteases and Delayed Wound Healing. Advances in Wound Care (New Rochelle). 2013 Oct;2(8):438-447. (5). Finnegan S, Percival SL. EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care. Adv Wound Care (New Rochelle). 2015 Jul 1;4(7):415–21. (6). Gawande G, LoVetri K, Yakandawala N, Froelich G, Madhyastha S. Antimicrobial-antibiofilm compositions and methods of use thereof [Internet]. Winnipeg, MB; US9980497B2, 2018. Available from: https://patents.google.com/patent/US9980497B2 (7). Visvalingam J, Yakandawala N, Regmi S, Lee SP, Sailer M. A Chelation approach to biofilm and metalloprotease problem in chronic wounds. In: The Symposium on Advanced Wound Care Spring. National Habor, BD, USA.; 2023. (8). Rodgers A, Watret L. The role of pH modulation in wound bed preparation. The Diabetic Foot. 2005;8(3):154–7. (9). Visvalingam J, Regmi S, Lee SP, Edwards M, Abdo R, Schultz G. A thermo-reversible hydrogel against chronic, burn and combat wound associated pathogens, and its application to colonized wounds. In: The Symposium on Advanced Wound Care Spring. Orlando, FL, USA; 2024. (10). Sailer M, Visvalingam J, Regmi S, Muzaleva A, Schultz G. A novel thermo-reversible antimicrobial hydrogel spray with effective antibiofilm properties to treat complex combat wounds. MHSRS, in Kissimmee FL, USA; 2024. (11). Hübner NO, Kramer A. Review on the Efficacy, Safety and Clinical Applications of Polihexanide, a Modern Wound Antiseptic. Skin Pharmacol Physiol. 2010;23(Suppl. 1):17–27. (12). Gray D, Barrett S, Battacharyya M, Butcher M, Enoch S, Fumerola S, et al. PHMB and its potential contribution to wound management. Wounds UK. 2010;6(2):40–6. (13). Rippon MG, Rogers AA, Ousey K. Polyhexamethylene biguanide and its antimicrobial role in wound healing: a narrative review. J Wound Care. 2023 Jan 2;32(1):5–20. (14). Percival SL, Chen R, Mayer D, Salisbury A. Mode of action of poloxamer‐based surfactants in wound care and efficacy on biofilms. Int Wound J. 2018 Jun 5;15(5):749–55. (15). Percival SL, Mayer D, Kirsner RS, Schultz G, Weir D, Roy S, et al. Surfactants: Role in biofilm management and cellular behaviour. International Wound Journal. 2019 Jun;16(3):753–60. (16). Visvalingam J, Yakandawala N, Regmi S, Sharma P, Sailer M. Anti-biofilm and anti-metalloprotease activity of a novel wound gel. In: The Symposium on Advanced Wound Care Fall. Las Vegas, NV, USA; 2022. (17). Visvalingam J, Sailer M, Edwards M, Schultz G. A Novel Thermo-Reversible Antimicrobial Hydrogel Spray to Treat Complex Combat Wounds. In: MTEC Annual Meeting. Baltimore, MD, USA.; 2024. (18). Visvalingam J, Yakandawala N, Regmi S, Adeniji A, Sharma P, Sailer M. Wound Gel Formulations Containing Poloxamer 407 and Polyhexanide Have In Vitro Antimicrobial and Antibiofilm Activity Against Wound-Associated Microbial Pathogens. Microorganisms. 2024 Nov;12(11):2362. (19). Data on file. * Anecdotal observation.